The Ph of a Solution Is Defined as
The pH of a solution is the negative logarithm base 10 of the activity the product of the molar concentration and the activity coefficient of the hydrogen ions H in the solution. Following is the equation that is used for calculating the pH.

Let S Talk Science A Ph Solution Washington State Department Of Ecology
Calculate the concentration of hydrogen ions in moles per liter M.

. PH -log 10 H. The term widely used in chemistry biology and agronomy translates the values of the concentration of the hydrogen ion which ordinarily ranges between about 1 and 10 14 gram-equivalents per litreinto numbers between 0 and 14. 2711pHlogHBecause the ion product of water Kw HOH 104 1014 at 25C it follows that a neutral solution eg pure water at 25C in which HOH has a pH7.
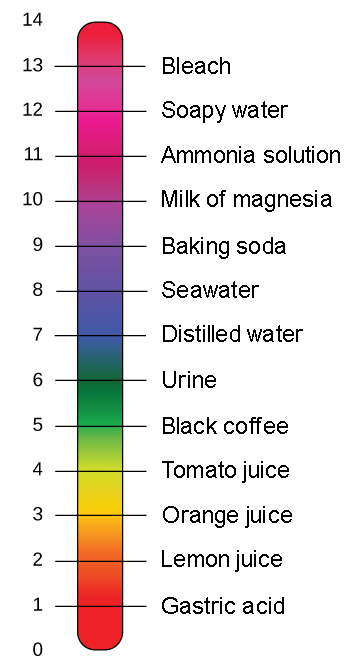
PH is defined as the measure of hydrogen ion concentration used to measure the acidity or alkalinity of a given solution. How would I carry this out. The pH scale usually ranges from 0 to 14.
In Chemistry the pH of a solution is defined by pH -log10H where H is the hydrogen ion concentration of the solution in moles per liter. The pH of a solution is defined as pH -logH3O. If the pH of a solution is 372- 003 what is the H3O and its absolute standard deviation.
Likewise the hydroxide ion molarity may be expressed as a p-function. PH is logarithmically and inversely related to the concentration of hydrogen ions in a solution. The pH is a measure of the concentration of hydrogen ions the acidity or alkalinity of a solution.
The pH of a solution is defined as the logarithm of the reciprocal of the hydrogen ion concentration or activity H of the solution. Of a solution is defined as the. PH is an abbreviation for power of hydrogen where p is short for the potenz the German word for power and H is the symbol of the element hydrogen.
It commonly ranges between 0 and 14 but can go beyond these values if sufficiently acidicbasic. PH is a measure of how acidic or basic a chemical solution is. PH - log H3O Or pH log 1 H3O Optikn D is correct.
The pH scale runs from 0 to 14a value of seven is considered neutral less than seven acidic and greater than seven basic. How do I do it the way they are asking for. The pH of a solution is also defined as the logarithm of the reciprocal of left rm H right ion concentration.
Causes the formation of additional hydronium ions and a resulting lower concentration of hydroxyl ions. Because K w is constant 10 times 10-14 at 25 C the pK w is 14 the constant of water determines the range of the pH scale. PH logH 3 O Rearranging this equation to isolate the hydronium ion molarity yields the equivalent expression.
The pH-scale is normally between 0 and 14. H 3 O 10 pH. The pH of a solution is defined as the _______ _______ of the hydrogen ion concentration.
H M B The pH of lemon juice is 20. The pH of a solution is the negative logarithm to the base 10 of the hydrogen ion concentration expressed in moles per litre. The pH of a solution is defined as the A negative logarithm class 12 chemistry CBSE.
To understand what the pK w is it is important to understand first what the p means in pOH and pH. Acid added to water. The pH of a solution is therefore defined as shown here where H 3 O is the molar concentration of hydronium ion in the solution.
PH quantitative measure of the acidity or basicity of aqueous or other liquid solutions. The letters pH stand for power of hydrogen and the numerical value is defined as the negative base 10 logarithm of the molar concentration of hydrogen ions. Idea of using letter p to denote concentrations and numbers that can vary by several orders of magnitude was widely accepted and is used not only in pH definition but for example also for displaying dissociation constants.
Bases added to water. The meaning of PH is a measure of acidity and alkalinity of a solution that is a number on a scale on which a value of 7 represents neutrality and lower numbers indicate increasing acidity and higher numbers increasing alkalinity and on which each unit of change represents a tenfold change in acidity or alkalinity and that is the negative logarithm of the effective hydrogen-ion. Logarithm of reciprocal of magnitude of hydrogen ion concentration.
Beer has a pH of 3 therefore its H concentration is ___ times greater than a solution with a pH of 5. Compounds that increase the concentration of negatively charged hydroxyl ions when dissolved in water. The Danish biochemist Søren Sørenson proposed the term pH to refer to the potential of hydrogen ion He defined the p as the.
PH is the negative base 10 logarithm log on a calculator of the hydrogen ion concentration of a solution. As a pH of a solution increases the concentration of ______ ions increases. PH -logH Values of pH.
Aqueous solutions at 25C with a pH less than 7 are acidic while those with a pH greater than 7 are basic or alkaline. Negative logarithm of the magnitude of hydrogen ion concentration. Q1 pH - pH of a solution is defined as the negative logarithm concentration of the hydronium ion.
I know to find the hydronium concentration wouldnt i just take e372. The pH to H formula that represents this relation is. Calculate the concentration of hydrogen ions in moles per liter M.
The range of the pH scale varies from 0 to 14. A year later at the same river the pH was measured as 141 x 10-8 molL. The pH scale for acidity is defined by pH log10 H where H is the concentration of hydrogen ions measured in moles per liter M.
The pH of a solution is a measure of the molar concentration of hydrogen ions in the solution and as such is a measure of the acidity or basicity of the solution. The measure of acidity or alkalinity of a solution. PH is a measure of hydrogen ion concentration a measure of the acidity or alkalinity of a solution.
At a river the pH was measured as 236 x 10 molz. Aqueous solutions at 25 C with a pH of less than 7 are acidic and basic or alkaline solutions are those with a pH greater than 7. A The pH of egg whites is 83.
It is much easier to use pH definition and to say pH of the solution is 41 than to use concentrations - as in H concentration is 0000079M. In solutions of low ionic strength pH can be defined as the negative logarithm of the molar concentration of the hydrogen ions because activity and concentration are nearly identical in these solutions. Q2 strong ac View the full answer.
The pH scale pH is a numeric scale which is used to define how acidic or basic an aqueous solution is.

The Ph Scale Chemistry For Non Majors

Comments
Post a Comment